what are the variables to keep in the final dataset in sdtm mapping
The SDTM standard is a CDISC standard, and it ways the Written report Data Tabulation Model. If you want to know more about CDISC standards you lot tin can read our introduction to CDISC standards, or you lot can read about how CDISC standards fit into the drug development process.
What is SDTM in clinical trials?
CDISC SDTM is the name of the model (or framework) used for organizing information collected in human being and brute clinical trials. The model was developed past CDISC – the Clinical Data Interchange Standards Consortium – a standards development organisation for dealing with medical research information.
Once you've gathered all the necessary data for your trial, it must be converted into a specific table format optimized for review, to be accepted by the FDA. The study data tabulation model – SDTM – is the name of that structure.
Why is SDTM required?
SDTM is there to give regulatory reviewers – namely the FDA – a clear description of the structure, attributes, and contents of each dataset, and the variables submitted as office of your clinical trial. Before CDISC SDTM was enforced, there were different domain names for each domain, different variables, and different variable names. Nothing was standard. As a result, reviewers spent huge amounts of fourth dimension trying to go the data into a standard format – figuring out the domain names and names of the variables in each dataset – rather than reviewing the data itself. This ultimately prolonged the clinical trial process.
Yous can read more about what other CDISC standards are required for regulatory submissions.
The introduction of CDISC SDTM standardized this terminate to end required data structure. Now we have standard domain names and a standard structure for each domain. At that place are standard variables and standard names for SDTM datasets. It ways that each bit of data collected tin can at present exist hands identified. Regulators can review the information much quicker, making the process far more efficient. Plus, it makes all of your studies consistent, considering they're all in the same standard format.
![]() Notation
Notation
Formalizing the structure of the domains has also led to the development of conformance rules, by both the CDISC SDTM team and regulatory bodies such equally FDA and PDMA. You can discover out about the FDA's clinical trial guidance documents. These rules are programmed into software validation tools to automate the checking of SDTM clinical datasets, against the conformance dominion.
What is the latest version of SDTM?
CDISC build the SDTM standards from ii important models:
- "Core" Study Data Tabulation Model.
- SDTM Implementation Guide (SDTM-IG).
The core model provides a standardized set of variables, assembled into "classes", which are refined and built into variable collections for specific uses cases (SDTM-IG domains), east.g Vital Signs observations, Medical History reporting, etc. The SDTM core model also supports the non-human trial standard SEND-IG.
The latest versions being SDTM v1.7 for SDTM-IG v3.3 and SDTM-IG Medical Devices (SDTMIG-Doctor) v1.i. CDISC is always developing new domains. It's important to regularly check the CDISC website for the latest updates.
There is currently a Public Review for SDTMIG v3.4, SDTM 2.0, and Conformance Rules v1.2. Comments are due past one June 2020. Read more about planned updates to standards.
Formedix's free guide on how to overcome SDTM implementation problems

What are SDTM domains?
In order to be able to correctly implement the SDTM, information technology's of import to have a skilful understanding of its domains and how they're structured.
SDTM is based on the observations that are collected from subjects taking role in a clinical trial. An observation is a piece of data nerveless during a study. For example "Subject field 12 had a balmy headache starting on study day 5".
Most observations nerveless should be classified into one of the full general ascertainment classes (too known as information classes). These are:
- Interventions
- Events
- Findings
Each of the general observation classes has associated domains. A domain is only a group of observations that share a common topic, such as Medical History or Vital Signs. In addition to the general observation classes, there are three other domains. Simply we'll get into the various SDTM domains after a bit more on variables.
The example below shows the observations "Nausea", "Headache" and "Dizziness". These are function of the Adverse Events domain. And the Agin Events domain is in the Events ascertainment form.
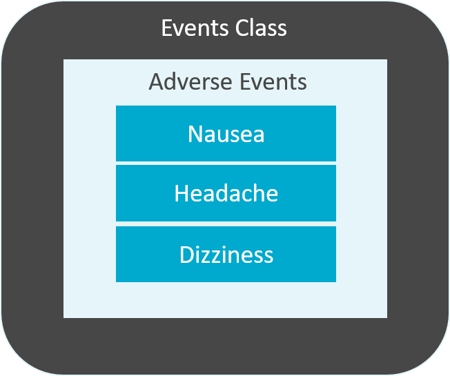
Domains are prefixed by a two-character domain code that's used to map a variable to a domain. For example, the domain 'Medical History' is prefixed past the domain lawmaking MH. The variable –SEQ contains two hyphens that bespeak a domain code is required. So the case becomes MHSEQ. Another example is the variable –TESTCD in the Vital Signs domain becomes VSTESTCD. Each domain has a dataset which is a collection of related data. SDTM datasets are described past a set of named variables. And each of these named variables is categorized by their role.
What are SDTM variable roles?
A role category conveys a particular type of information virtually a variable. And variables can have just one role.
Variable roles take 5 categories:
- Identifier variables allow the written report, subject, domain and sequence number of a record to exist identified.
- Topic variables depict the focus of an observation.
- Timing variables depict the date, time, and duration of an observation.
- Qualifier variables draw the results of an ascertainment with text or numeric values.
- Rule variables describe algorithms or methods for calculations or looping conditions and are mainly used for the Trial Design domain.
In the example below, variable roles are shown in the superlative row of the table. The color-coded areas on the second row show the variables that represent to the variable roles.
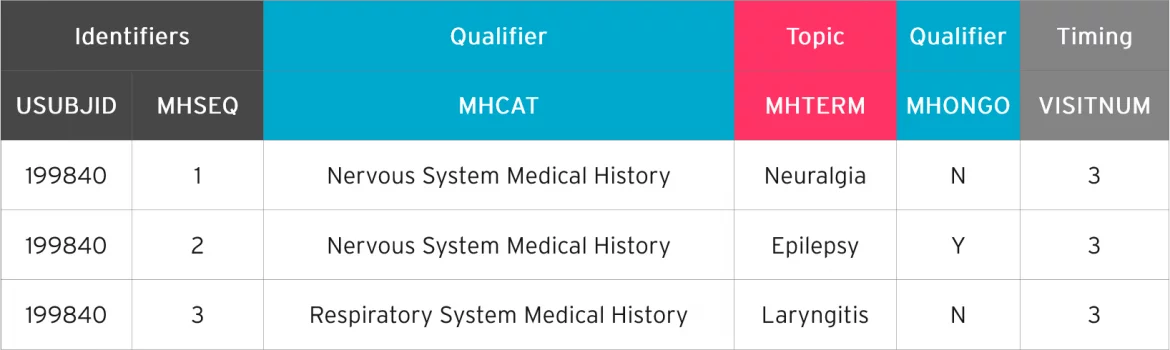
Qualifier variables are further categorized as follows:
- Grouping qualifiers group observations together.
- Result qualifiers draw the result for a finding.
- Synonym qualifiers comprise some other proper noun for the observation.
- Record qualifiers define the supplementary attributes of an observation.
- Variable qualifiers describe the value of an ascertainment.
What are SDTM core variables?
Cadre variables are a measure of compliance with the specific SDTM-IG domain model. The value of a core variable shows the importance of the variable to the overall domain structure.
Variables are divided into three categories:
- Required variables are needed to place a data record, eastward.g STUDYID, and USUBJID. Or, they are needed to make a tape easily understood, eastward.g TERM and TEST. They must e'er be included in the dataset and cannot be null.
- Expected variables are needed to make a record useful within a specific domain. They must always be included in the dataset but they tin can be null for some records. If no data is collected, a annotate must exist included to explain why.
- Permissible variables must be included in the dataset if results are collected or derived, only they can be left nix or blank.
Variables from the parent class tin also exist inserted into the domain if required.
What SDTM domains are there?
Currently, there'south a big collection of domains, and CDISC is constantly developing more than. These consist of names, with abbreviations. For case, Demographics (DM), Subject field Visits (SV), Adverse Events (AE), Lab Results (LB), and Vital Signs (VS) to proper name a few. Each SDTM domain usually consists of a file, named afterwards the domain (e.k AE.xpt).
Most observations that are collected fit into 1 of the general observation classes:
- Interventions datasets capture treatments and procedures that are given to a field of study as specified by the protocol. Examples are Exposure (EX), Concomitant Medications (CM), and Substance Use (SU), due east.thou. tobacco, caffeine, alcohol.
- Events datasets capture planned protocol milestones such equally randomization and study completion. Unplanned incidents that occur earlier, or during a written report are also captured. Examples are Agin Events (AE), Disposition (DS), and Medical History (MH).
- Findings capture observations that accost specific questions such equally observations made during physical examinations, laboratory tests, ECG testing, etc. Findings About is included and captures data related to the Interventions and Events classes. Examples are Vital Signs (VS), Physical Examination (PE), Labs (LB), and Subject Characteristics (SC).
In addition to the general observation classes, there are iii special case classes:
- Special Purpose datasets tin can exist Demographics (DM), Comments (CO), Subject Elements (SE), and Subject Visits (SV).
- Trial Design has datasets that describe the design of a trial. Examples are Trial Summary (TS), Trial Artillery (TA), and Trial Visits (TV).
- Relationship datasets represent the relationships between datasets and records.

How to implement SDTM
The following section explains how to map source datasets to SDTM domains, considerations, and other necessary deliverables.
How to do an SDTM mapping
The SDTM-IG extends and refines the SDTM core model with specific domain implementations, business rules, assumptions, and examples. It should exist used forth with the relevant version of the SDTM. So make sure y'all have the correct versions of both of these documents.
Here are some basic steps to help keep y'all on the right rail:
- Decide which SDTM domains to create.
- Compare the SDTM metadata to the SDTM metadata and map directly where possible.
- Map the rest of the source datasets to SDTM domains.
- Map variables in the source datasets to the variables in the SDTM domains.
- Decide whether custom domains and SUPPQUAL domains need to be created.
- Perform the data conversion – there are diverse mapping tools you tin can use to do this.
- Validate the SDTM datasets.
- Generate and validate Define.xml.
There are a number of different types of SDTM mappings y'all tin do for steps 2, 3, and iv above.
- Direct map to a domain variable without making any changes.
- Rename the source variable name and label without the demand to brand any other changes.
- Map values to standard units or terminology.
- Alter the format of a source variable.
- Combine two or more than source variables to brand a single domain variable.
- Carve up a single source variable into two or more than domain variables.
- Derive a domain variable from i or more than source variables using logic, computation, algorithm or decoding.
And remember, you might need to use more than one blazon of mapping to create an SDTM variable.
SDTM mapping can exist a complicated job, so it's important to plan everything out in advance. By creating a mapping specification, you lot'll know where data came from, how information technology came and where it'southward to go to. There are various mapping scenarios you tin can use. It's important to utilize the SDTM model and Implementation Guide during this procedure. And by using standard process and tools, you'll maximize your chances of success.
SDTM mapping specifications should be adult at the same time as annotating CRFs. The mapping specification tells the user how to do a mapping. An annotated CRF is a visual representation of a mapping showing how the source data relates to the SDTM data.
If this sounds similar a lot to take on, there'southward some neat technology that tin can help to automate this process. Come across how SDTM conversion can be much quicker and easier with our SDTM mapping tools and SDTM automation.
FREE Guide: SDTM mapping process How-To, typical scenarios & all-time practices
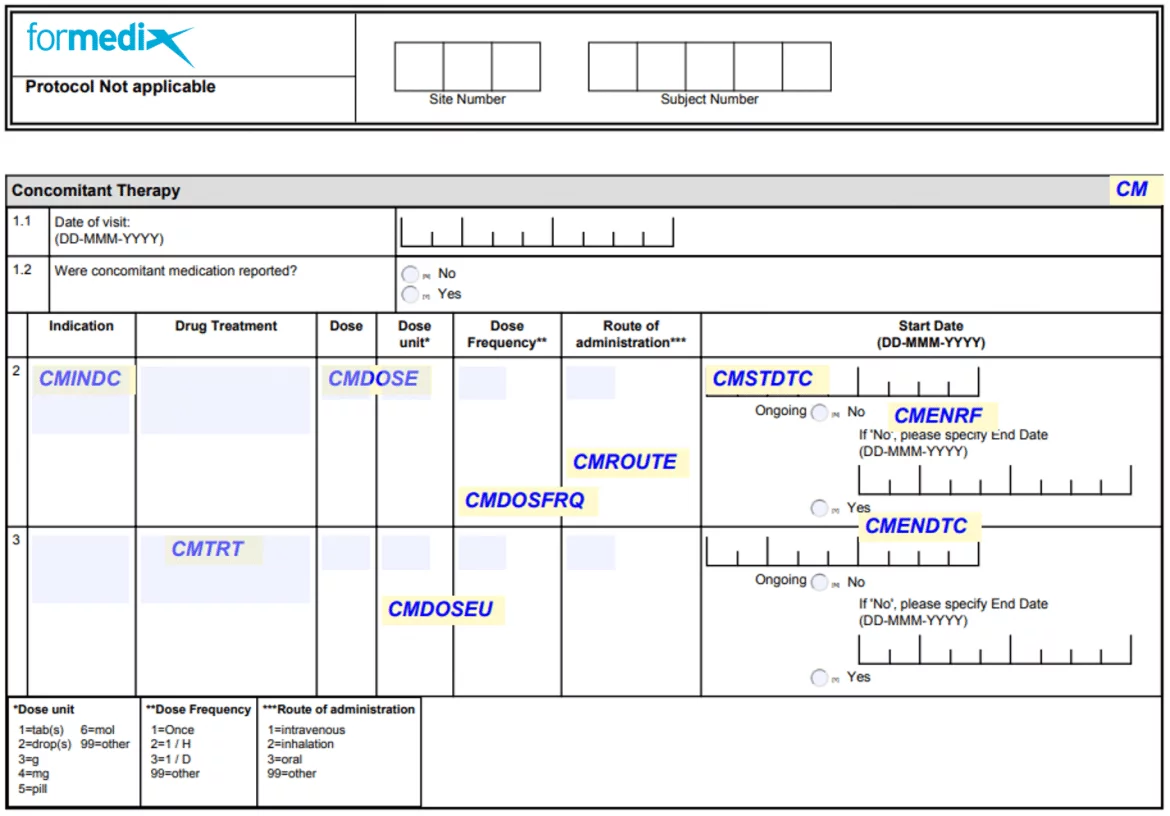
SDTM annotated CRFs
A Bare CRF is a collection of pages that is a mandatory deliverable for submission to the FDA. The file is e'er called blankcrf.pdf. Each question on a class must exist manually annotated to evidence the origin of variables. It links the fields on the form with the variables in the dataset (the source of the information). Annotations help the reviewer observe where variables come from in the submitted SDTM datasets. Observe out more virtually the benefits of automating annotated CRFs.
What is SDTM controlled terminology?
SDTM has standard codelists for particular variables with allowable values for these variables. These values are required for submission to the FDA and PMDA in CDISC complaint SDTM datasets. Yous should always use the most up to appointment version of controlled terminology when you get-go to map your SDTM datasets. Find out more about using NCI controlled terminology for standardizing data.
CDISC and NCI Enterprise Vocabulary Services partnered up to develop a standard controlled terminology. However, the CDISC / NCI controlled terms for Lab tests are not unique. They crave additional information for differentiation.
Other medical dictionaries tin can be used, such every bit MedDRA and WHOdrug.
SDTM datasets and LOINC codes
Over the last 25 years, the LOINC projection has provided a standard classification for health measurements. Most SDTM programmers will encounter "LOINC Lawmaking" information in Lab data. But the classification system has been extended to cover other measurements such as ECG. And so what is LOINC? LOINC is an internationally recognized classification system and is often requested in regulatory information submissions to provide context to clinical measurement data, e.thousand. Labs and ECG. Read more hither most LOINC codes and SDTM.
SDTM Define-XML
The FDA requires a Define.xml file to exist included for all drug submissions. It describes the content and structure of data nerveless during the study. The Ascertain.xml file makes the review of study data quicker and easier for the FDA. Yous can read our web log almost using the Ascertain XML standard for dataset blueprint.
View our free guide on how to overcome common difficulties complying with Define-XML
The latest version of the standard is Ascertain two.0. Information technology describes the content and structure of data collected during the written report which are domains, variables, methods, controlled terminology, and supporting documents. I of the things that crop up oft is how to handle data coming in from multiple sources. You can read our web log on how to depict multiple origins for a value in Define-XML 2.0.
 Creating a ascertain.xml requires a lot of programming expertise. It takes a lot of time. That'south why it's then important to make the procedure equally quick and piece of cake as possible.
Creating a ascertain.xml requires a lot of programming expertise. It takes a lot of time. That'south why it's then important to make the procedure equally quick and piece of cake as possible.
Detect out more than >
How Formedix can help…
At that place'due south a lot to get your caput around! Simply, did yous know we're on the CDISC XML technical team? We were involved in creating the CDISC ODM and Define models. We've been in the business for over 20 years. And then our CDISC knowledge isn't also bad! Learn more well-nigh how we help yous with CDISC Compliance. And, we're well placed to give real-world, practical CDISC training.
Our clinical metadata repository and clinical trial automation software support all versions of CDISC standards and SDTM automation. We proceed our platform updated in line with CDISC and NCI standards. That way your report designs and datasets are always regulatory compliant.
You can get in touch with us by booking an online customized demo or you can request a telephone call with us. Just tell united states what date and time suits, and how we tin can assistance.
And, you lot have the option of a gratis trial. It comes with vi hours of grooming to get y'all started!

Source: https://blog.formedix.com/all-you-need-to-know-about-sdtm
0 Response to "what are the variables to keep in the final dataset in sdtm mapping"
Post a Comment